Drugmaker shut down after black schmutz found in injectable weight-loss drug
The Food and Drug Administration is warning consumers not to use any drugs made by a compounding pharmacy in California after regulators realized the pharmacy was making drugs that need to be sterile—particularly injectable drugs—without using sterile ingredients or any sterilization steps.
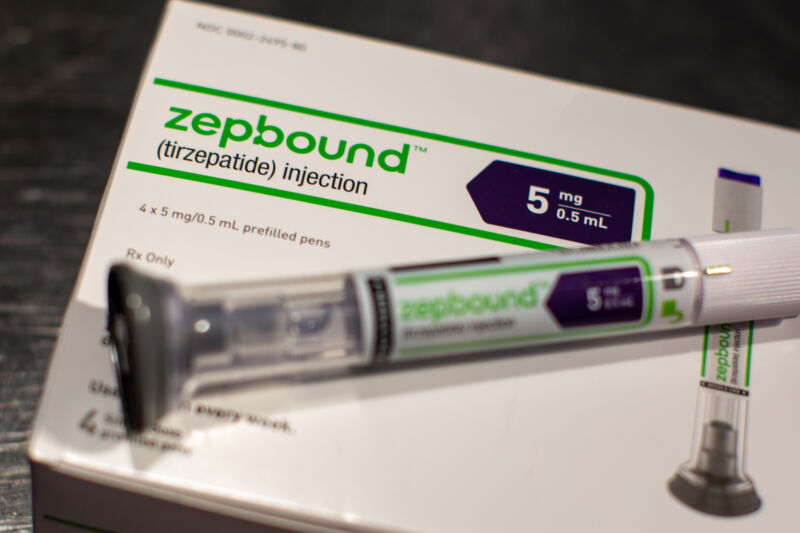
The products made by the pharmacy, Fullerton Wellness LLC, in Ontario, California, include semaglutide, which is intended to mimic brand-name weight-loss and diabetes drugs Wegovy and Ozempic. Fullerton also made tirzepatide, which is intended to mimic weight-loss and diabetes drugs Zepbound and Mounjaro.
The FDA became aware of the problem after a patient submitted a complaint to the regulator that a vial of semaglutide from Fullerton Wellness had an unidentified "black particulate" floating in it. Semaglutide, like tirzepatide, is injected under the skin and is intended to be sterile.

© Getty | Steve Prezant