Shady drugmaker used code words to sell knockoff weight-loss drug: Lawsuit
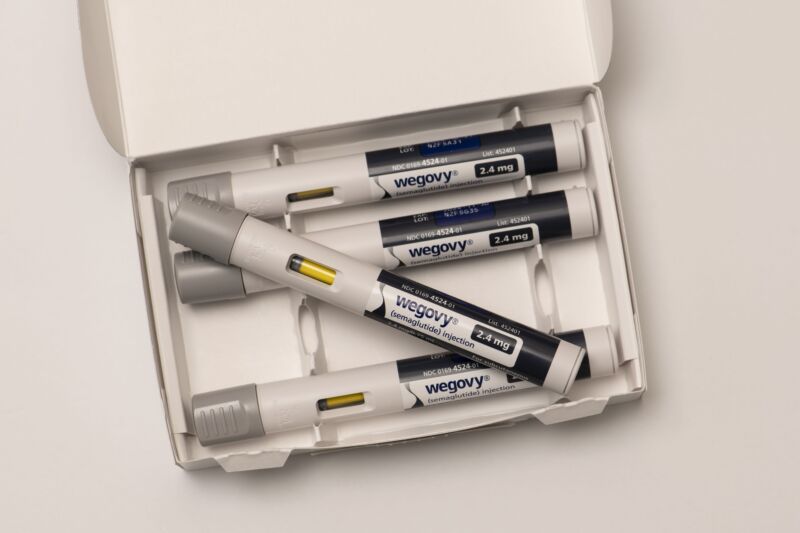
Amid ongoing legal battles over coveted GLP-1 therapies, a drug vendor in Washington state is accused of running an outlandish scheme to sell do-it-yourself kits to make illicit knockoff versions of weight-loss and diabetes drugs, Zepbound and Mounjaro.
For the alleged scheme, vendor Pivotal Peptides has customers buy a set of ingredients they have to mix together to create their own injectable versions of the drugs. Customers don't need a prescription or even a medical consultation to order the kit, even though the brand-name drugs are prescription-only. That may not be surprising, though, since the dubious white powder customers receive is stated to be "a research chemical for lab research and veterinary purposes only." Once purchased, the kit's instructions recommend users disinfect their home work surface before beginning and stress the importance of using the sterile water included in the kit to dissolve the powder to the desired concentration. The instructions then explain how to inject oneself with the homemade mixture using a 30-gauge syringe.
That's all according to a lawsuit filed Monday by pharmaceutical giant Eli Lilly, maker of tirzepatide-based Zepbound and Mounjaro, which are sold as ready-to-use medicines in single-dose pens or vials. The lawsuit against Pivotal Peptides is one of three that Lilly filed this week, all accusing questionable drugmakers of unlawfully selling knockoff versions of its tirzepatide drugs that have not been tested or approved. But the one against Pivotal Peptides stands out for the scheme the owners allegedly used to sell their knockoff version.
© Getty | Stefan Cristian Cioata